| Active Ingredient | BOSUTINIB MONOHYDRATE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BOSULIF | (NDA) 203341 | WYETH PHARMS INC | TABLET;ORAL | EQ 100MG BASE, EQ 500MG BASE | EQ 100MG BASE (RS) | September 4, 2012 | September 4, 2017 | September 4, 2019 | 1 New molecular entity (NME) | S Standard review drug O Orphan drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
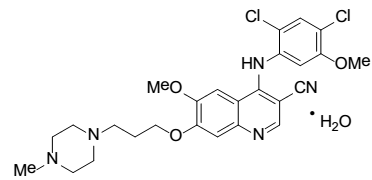 |
| Chemical Name | 3-Quinolinecarbonitrile, 4-[(2,4dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl) propoxy]-, hydrate (1:1) |
| CAS No | 380843-75-4 |
| Molecular Formula | C26H29Cl2N5O3•H 2O (monohydrate) |
| Molecular Weight | 548.46 (monohydrate), equivalent to 530.46 (anhydrous) |
| Appearance | a crystalline white to yellowish tan powder |
| Solubility | Bosutinib monohydrate has a pH dependent solubility across the physiological pH range. At or below pH 5, bosutinib monohydrate behaves as a highly soluble compound. Above pH 5, the solubility of bosutinib monohydrate reduces rapidly. It is soluble in acetone, methylethyl ketone, 2-propanol, ethyl acetate, methyl isobutyl ketone, acetonitrile, methanol; sparingly soluble in isopropyl acetate; and slightly soluble in toluene and heptanes. |
| Water Solubility | - |
| Polymorphism | Since bosutinib monohydrate (Form 1) is the thermodynamically favoured solid state form, this form was selected for development and commercialisation. |
| pKa (Strongest Acidic) | 15.48 (Predicted) |
| pKa (Strongest Basic) | 8.43 (Predicted) |
| Log P | - |
| Identification | IR, HPLC |
| Degradation | - |
| Hygroscopic | non-hygroscopic |
| Photostability study | not light sensitive |
| Melting Point | - |
| BCS Class | IV (Permeability studies conducted in accordance with the BCS classification guidance supported the classification of bosutinib as a low permeability drug) |
| Manufacture of API | Bosutinib is supplied by one active substance manufacturer. It is synthesised in several steps using commercially available well defined starting materials. The manufacturing process conditions have been designed to robustly and reproducibly produce the monohydrate (Form 1). |
| Parameters | Details |
|---|---|
| Indications and Usage | BOSULIF is indicated for the treatment of adult patients with chronic, accelerated, or blast phase Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML) with resistance or intolerance to prior therapy. |
| Dosage and Administration |
The recommended dose and schedule of BOSULIF is 500 mg orally once daily with food. Continue treatment with BOSULIF until disease progression or patient intolerance. If a dose is missed beyond 12 hours, the patient should skip the dose and take the usual prescribed dose on the following day. Dose Escalation: Consider dose escalation to 600 mg once daily with food in patients who do not reach complete hematological response (CHR) by week 8 or a complete cytogenetic response (CCyR) by week 12, who did not have Grade 3 or higher adverse reactions, and who are currently taking 500 mg daily. |
| Mechanism of action | Bosutinib is a tyrosine kinase inhibitor. Bosutinib inhibits the Bcr-Abl kinase that promotes CML; it is also an inhibitor of Src-family kinases including Src, Lyn, and Hck. Bosutinib inhibited 16 of 18 imatinib-resistant forms of Bcr-Abl expressed in murine myeloid cell lines. Bosutinib did not inhibit the T315I and V299L mutant cells. In mice, treatment with bosutinib reduced the size of CML tumors relative to controls and inhibited growth of murine myeloid tumors expressing several imatinib-resistant forms of Bcr-Abl. |
| Absorption | Following administration of a single dose of BOSULIF 500 mg with food in patients with cancer, the median timeto-peak concentration (t max ) was 4-6 hours. Bosutinib exhibits dose proportional increases in AUC and Cmax , over the dose range of 200 to 800 mg. After 15 daily doses of BOSULIF (500 mg) with food in patients with CML, the mean (SD) Cmax value was 200 (12) ng/mL, and the mean (SD) AUC was 3650 (425) ng•h/mL. |
| Food Effect | When given with a high fat meal, the Cmax and AUC of bosutinib increased 1.8- and 1.7-fold, respectively. |
| Distribution | After administration of a single dose of BOSULIF 500 mg with food in patients with CML, bosutinib had a mean apparent volume of distribution ± standard deviation of 6080 ± 1230 L. Bosutinib was highly bound to human plasma proteins in vitro (94%) and ex vivo in healthy subjects (96%), and binding was not concentration-dependent. |
| Metabolism | Bosutinib is primarily metabolized by CYP3A4. The major circulating metabolites identified in plasma are oxydechlorinated (M2) bosutinib (19% of parent exposure) and N-desmethylated (M5) bosutinib (25% of parent exposure), with bosutinib N-oxide (M6) as a minor circulating metabolite. All the metabolites were deemed inactive. |
| Elimination |
In patients with CML given single oral doses of BOSULIF 500 mg with food, the mean terminal phase elimination half-life (t 1/2 ) was 22.5 (1.7) hours, and the mean (SD) clearance (Cl/F) was 189 (48) L/h. In six healthy male subjects given a single oral dose of [14C] radiolabeled bosutinib, 91.3% of the dose was recovered in feces and 3% of the dose recovered in urine. |
| Peak plasma time (Tmax) | 4 to 6 hours |
| Half life | 22.5 (1.7) hours |
| Bioavailability | - |
| Age, gender | - |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 30218 | A | II | February 5, 2016 | MSN LABORATORIES PRIVATE LTD [ROUTE CODE BS] |
| 30278 | A | II | February 26, 2016 | MSN LABORATORIES PRIVATE LTD (Form R) |
| 30683 | A | II | July 8, 2016 | MSN LABORATORIES PRIVATE LTD (FORM-I) |
| Parameters | Details | ||
|---|---|---|---|
| Strength | 103.40 mg of bosutinib monohydrate, equivalent to 100 mg of bosutinib | 516.98 mg of bosutinib monohydrate, equivalent to 500 mg of bosutinib | |
| Excipients used | microcrystalline cellulose, croscarmellose sodium, poloxamer, povidone, magnesium stearate | ||
| Composition of coating material | polyvinyl alcohol, titanium dioxide (E171), macrogol 3350, talc (E553b) and iron oxide yellow (E172) | polyvinyl alcohol, titanium dioxide (E171), macrogol 3350, talc (E553b), iron oxide red | |
| Composition of caspule shell | - | ||
| Pharmaceutical Development | Excipients were selected: microcrystalline cellulose as a diluent and compression aid, croscarmellose sodium as a disintegrant, poloxamer 188 as a binder and a olubilising/wetting agent, povidone as a binding agent and magnesium stearate as a lubricant to develop immediate release tablet | ||
| Manufacture of the product | The manufacturing process has been validated by a number of studies for the major steps of the manufacturing process and has been demonstrated to be capable and to be able to reproducibly produce finished product of the intended quality. The in process controls are adequate for this tablets preparation. | ||
| Tablet / Capsule Image |

|

|
|
| Appearance | yellow, oval, biconvex, film-coated tablets debossed with “Pfizer” on one side and “100” on the other. | red, oval, biconvex, film-coated tablets debossed with “Pfizer” on one side and “500” on the other. | |
| Imprint code / Engraving / Debossment | debossed with “Pfizer” on one side and “100” on the other. | debossed with “Pfizer” on one side and “500” on the other. | |
| Score | no score | no score | |
| Color | YELLOW | RED | |
| Shape | OVAL Biconvex | OVAL Biconvex | |
| Dimension | 11mm | 18mm | |
| Mfg by | Pfizer Lab (EU) | ||
| Mfg for | - | ||
| Marketed by | - | ||
| Distributed by | Pfizer Lab (US, EU) | ||
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N203341 | 1 | 6002008 | March 27, 2018 | Y | Y | U - 1284 | - | Download |
| N203341 | 1 | 7417148 | January 23, 2026 | - | - | U - 1283 | - | Download |
| N203341 | 1 | 7767678 | November 23, 2026 | Y | Y | - | - | Download |
| N203341 | 1 | 7919625 | December 11, 2025 | - | Y | - | - | Download |
| N203341 | 1 | RE42376 | September 24, 2019 | Y | - | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 50 | 0.1 N HCl | 900 | 10, 15, 20, 30 and 45 | June 25, 2015 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | BOSULIF | Download |
| UK | BOSULIF | Download |
| US | BOSULIF | Download |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
